
Prof Sue Walker AO
MBBS MD FRANZCOG CMFM DDUSue is a maternal fetal medicine specialist and Head of the Department of Perinatal Medicine at the Mercy Hospital for W...
Our genetic material, or DNA, is organised into 46 packages called chromosomes. Large changes that cause gains or losses of chromosomes are referred to as chromosomal conditions. Every baby has a small chance of being born with a chromosomal condition, the most common of which is Down syndrome.
The aim of prenatal screening is to offer a safe, accessible test to identify women at increased chance of having a baby with a chromosome condition. Prenatal screening for Down syndrome has been an established part of antenatal care for several decades. Women who are identified as having an 'increased chance' of a fetal chromosome condition by a screening test are then offered an diagnostic test, such as amniocentesis, for confirmation.
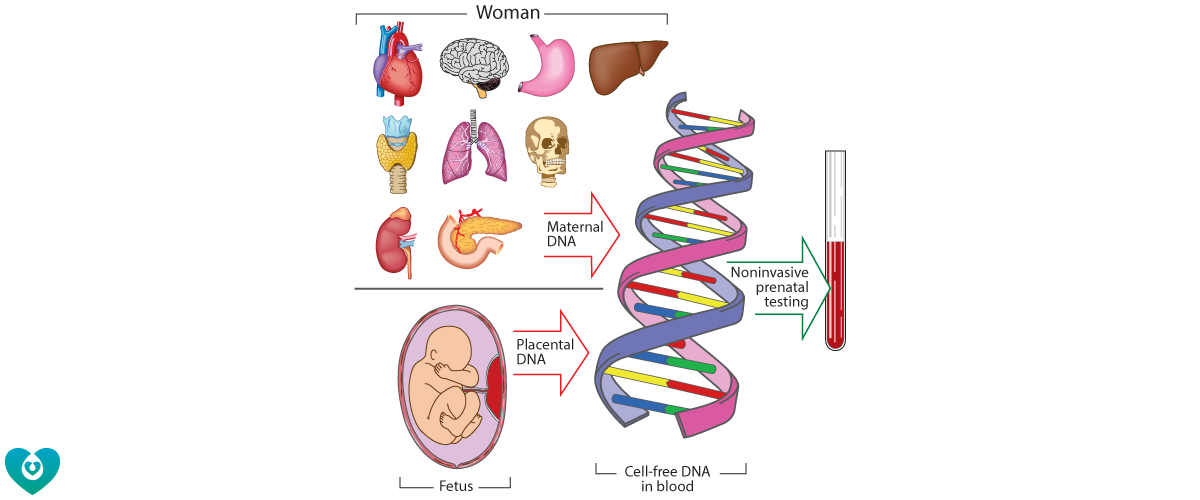
Our concept of genetic testing has advanced dramatically since it was discovered that DNA fragments from placenta are detectable in the pregnant woman's blood. We are now able utilize this uniquely accessible genetic material from the placenta to check the health of the developing fetus with a simple blood test, so called 'noninvasive prenatal testing' (NIPT). While this has dramatically reduced the need for invasive testing during pregnancy, it has created many new medical, ethical and workforce issues. We are still learning about the biology of cell-free DNA and what it can tell us about the health of mother and baby.
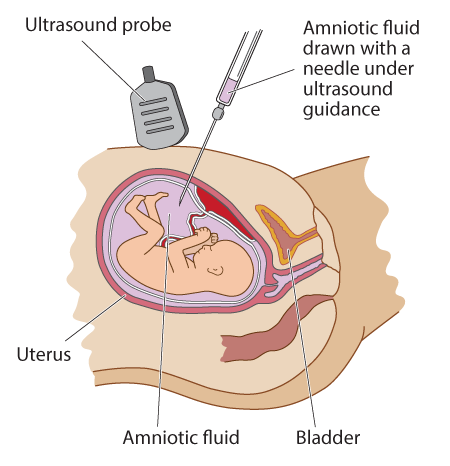
Each year, more than 1500 pregnant women in Victoria have an amniocentesis or CVS due to a possible chromosome condition in their unborn baby. Modern genomic testing with chromosomal microarrays can provide women with useful information about their unborn baby, but can also detect new genetic variants that are not well understood and consequently raise major concerns about the child's long term development.
The National Health Genomics Policy Framework has articulated essential principles for introducing genomics in health care – that it should be supported by evidence, be socially responsible, and person-centred. There is a clear need to improve the quality of fetal genomic information as it currently unclear whether providing information about all genetic changes is helpful or harmful to families.
We have partnered with the Reproductive Epidemiology group lead by Professor Jane Halliday at the Murdoch Children's Research Institute to study the rapidly changing environment of prenatal testing.
In our recently completed Perinatal Record Linkage (PeRL) study we analysed state-wide data on prenatal diagnosis, serum screening and NIPT to assess the real-world performance of prenatal screening in Victoria. We also investigated the relationship between socioeconomic disadvantage and the utilization of NIPT, and measured the prevalence of chromosome conditions during pregnancy and in infants.
We are now embarking on an NHMRC-funded cohort study to determine the outcomes of children who were diagnosed before birth with a genetic variation of "uncertain significance". The PALM (PrenataL Microarray) cohort study will help to close critical knowledge gaps through state-wide, multidisciplinary, comprehensive follow-up of children with genetic variations discovered before birth.
We gratefully acknowledge the support of our funders for this research theme, including the NHMRC, the Murdoch Children's Research Institute, the Norman Beischer Medical Research Foundation and Mercy Health.
